A naturalistic study of transcranial magnetic stimulation (TMS) in major depressive disorder
Saxby Pridmore 1,2 Sheila Erger 1, Yvonne Turnier-Shea 1, Tamara May 3
1 TMS Department, Saint Helen’s Hospital, Hobart, Tasmania, Australia; 2 School of Medicine, University of Tasmania, Hobart, Tasmania, Australia; 3 School of Psychology, Deakin University, Burwood, Victoria, Australia
Corresponding author: Prof S Pridmore, e-mail: s.pridmore@utas.edu.au
Received: 29/12/2019; revised 5/2/2020; Accepted: 25/2/2020
[citation: Pridmore, Saxby.; Erger, Sheila.; Turnier-Shea, Yvonne.; May, Tamara. (2020). A naturalistic study of transcranial magnetic stimulation (TMS) in major depressive disorder. DHH, 7(1):http://www.journalofhealth.co.nz/?page_id=2052].
Abstract
Background: TMS is effective in the treatment of major depressive disorder (MDD) in placebo-controlled trials and naturalistic out-patient studies. In Australia, TMS receives no rebate (currently), thus, privately insured patients generally receive this treatment as inpatients. Aim: A naturalistic study of the effects of TMS on depressive episodes of MDD in private hospital inpatients, to determine whether TMS is effective in severe depression, and whether age or gender impact on outcome. Method: Pre- and post-treatment HAMD6, visual analogue scale (VAS6) for depression, and CGI-S. In addition, a mid-treatment VAS6. Patients were grouped according to HAMD6 severity (mild, moderate, severe). Treatment: daily, 10 Hz, 4 second trains, a total of 75 trains at 110% resting motor threshold applied to the LDLFC. A course of treatment – 20 daily sessions over 26 days. Repeated measures ANCOVA were used to understand the impact of time, gender and age on depression levels.
Results: 52 patients with MDD participated – 35% had previously received TMS. Overall, 69% achieved remission – including 63.2% (12/19) of those with severe depression. Neither age nor gender impacted on outcome. Conclusion: A naturalistic inpatient study of TMS treatment of MDD produced remission in 69% of 52 patients – including 63.2% (12/19) with severe depression. As 35% of patients had received TMS previously, the results were better than might otherwise be expected. Neither age nor gender impacted on outcome.
Key words: Transcranial magnetic stimulation, depression, treatment resistant depression
Introduction
Major depressive disorder (MDD) is common, painful and difficult to treat. One third of patients do not respond to the first antidepressant administered (1), and one third do not benefit from adequate trials of known antidepressant medication (2,3).
Transcranial magnetic stimulation (TMS) is effective in treatment resistant MDD, as demonstrated in randomized placebo-controlled trials (4,5) and confirmed by meta-analyses (6,7). It is recommended as a treatment for MDD in the guidelines of many professional bodies (8,9).
The clinician is concerned with the likelihood of improvement of individual patients. In randomized placebo-controlled trials of TMS in MDD, 1) Levkovitz et al (10) used an H-coil (which likely stimulates more extensively than the figure eight coil) and reported at 5 weeks, remission rates of 32.6% for actively treated, and 14.6% for sham treated patients, 2) George et al (5) reported at 3 weeks, remission rates of 14.1% for actively treated, and 5.1% for sham treated patients, and 3) O’Reardon et al (4) reported at 6 weeks, the remission of 17.4% for actively treated, and 8.2% of sham treated patients.
With the antidepressant effects of TMS demonstrated in academic studies, the question becomes, is it useful in the real world, where patients suffer comorbidities, use substances, experience suicidal thoughts and are already consuming psychiatric medication? Naturalistic outpatient studies include, 1) Carpenter et al (11), 307 patients (42 US TMS services), reported objective remission in 37.1%, and subjective remission in 28.7-26.5%, 2) Galletly et al (12), 65 patients (Adelaide, Australia) reported remission in 28%, and 3) Rostami et al (13), 248 patients (Tehran, Iran) reported response in 45% of patients.
In Australia, there is currently no reimbursement for TMS treatment. Thus, in private practice, unless there is a benefactor (as in the Galletly et al (12) study), insured patients must deteriorate to the point of requiring hospitalization, so that the cost of treatment can be recovered from the hospitalization rebate. Saint Helen’s Private Hospital (Hobart, Australia) has been providing TMS treatment since 2004, and routinely collects clinical data using standardized instruments.
Some reports indicate younger people have a better response (14), but, others find age has no influence (13, 15). The impact of gender on outcome remains unexplored (16).
A few comparisons of ECT and TMS have indicated ECT has a stronger antidepressant effect (17). This has led to uncertainty about the efficacy of TMS in severe MDD.
The aim of this study was to determine whether TMS is useful in the treatment of MDD suffered by private psychiatric hospital inpatients. We determined the effect of TMS on the total MDD population, and on sub-populations of mild, moderate and severe depression. We also examined whether age or gender impact on outcome.
Method
The protocol and conduct of the study, and the publication of this report was sanctioned by the institutional ethics committee.
The study conducted through 2017.
Subjects were inpatients at a private psychiatric hospital, all suffering MDD and receiving antidepressant medication. All to have failed to respond to at least two courses of different antidepressants at appropriate doses administered for appropriate periods (thus, qualifying as treatment resistant MDD, or TRD (3)). Exclusion criteria: a history of epilepsy or currently pregnant, metal objects in the head or pacemaker in the chest, currently abusing or withdrawing from substances.
Treatment: Stimulation was provided by a MagPro device with a MagVenture Cool-B65 coil. Daily – 10 Hz, 4 second trains, 26 second inter-train interval, a total of 75 trains, at 110% resting motor threshold, applied 6 cm anterior to the site of the resting motor threshold on the left side. A course of treatment was 20 daily sessions over 26 days, with a 2-day rest period after each 5 treatments.
Routinely applied metrics: before and on completion of treatment: 1) the six-item Hamilton Depression Rating Scale (HAMD6) (18), 2) a six-item visual analogue scale (VAS6) (19), and 3) the Clinical Global Impression of Severity (CGI-S) (20). In addition, mid-way through the course (after 10 treatments) a further VAS6 was administered.
The HAMD6 items examine, 1) depressed mood, 2) work and energy, 3) somatic symptoms, 4) feelings of guilt, 5) anxiety (psychic), and 6) retardation.
The subjective VAS6 was designed to complement the objective HAMD6. Accordingly, the anchor points at either end of 10 cm lines: No depression – Worst possible depression; Activities give normal pleasure – Activities give no pleasure; No physical health concerns – Extreme health concerns; No feelings of guilt – Extreme feelings of guilt; and Not anxious – Most anxious possible. The 6th HAMD6 item concerns ‘retardation’ – in an inexact match we chose VAS6 anchor points: No concentration problems – Most possible concentrations possible.
The term, ‘remission’, is applied when symptom levels are low or absent (21), and is operationalized as HAMD6 score of <4 (22-24). ‘Partial remission/relapse – residual symptoms’ has been described (1,21) as an intermediate state, which can be operationalized as HAMD6 scores of 5 and 6. ‘Relapse’ describes the return of a depressive episode, after a period of remission (21,23); operationalized as a HAMD6 score of >7 (22,25).
Bech et al (22) equate a HAMD6 score of 7-8 with ‘mild ICD-10 depression’, 9-11 with
‘moderate ICD-10 depression’ and 12 and above with ‘severe ICD-10 depression’. We compared the admission and discharge HAMD6 scores for the total sample, and for these three depression levels.
Data Analyses
Descriptive statistics were used to describe participants and the number of participants at the different depression severity levels before and after treatment. Repeated measures ANCOVA was used with time as the repeated measure, gender as the between subject factor, covaried for age to understand if there were significant changes in symptoms with time, between gender and whether age was associated with symptom change. We also explored whether prior treatment (ECT or TMS) was associated with symptom change.
Results
Participant Characteristics
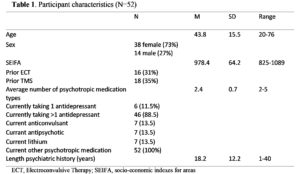
Over the year of the study, a total of 52 individuals completed a course of treatment, and 6 experienced relapse and completed a second course – for a total of 58 courses of treatment. Only the first courses of treatment were used in the following analyses. Table 1 shows the demographic characteristics of the participants. On average, the participants had over 18 years of psychiatric history, were taking over 2 types of psychotropic medications, 31% had previously received electroconvulsive treatment and 35% had previously received TMS treatment (but not in the preceding 6 months).
Neighbourhood socioeconomic disadvantage was measured using the Socio-Economic
Indexes for Areas (SEIFA) Disadvantage Index corresponding to the participant’s postcode of residence (Australian Bureau of Statistics, 2013). The SEIFA of participants was slightly below the Australian average (M=1000, SD=100), t(51)=-2.34, p=0.02.
Table 1. Participant characteristics (N=52)
Pre/Post Outcomes
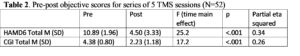
Repeated measures ANCOVA with Time as the repeated measure and gender as the independent variable covaried for age showed a significant reduction in HAMD6 and CGI Total scores post treatment (see Table 2). There was no main effect of gender on HAMD6 or CGI scores, age was not a significant factor in the analyses, indicating neither age nor gender impacted on outcomes of depression from the acute TMS sessions. The analyses were re-run to explore any effects of prior ECT or TMS treatment. There were no significant main effects of these prior treatments on the HAMD6 or CGI over time.
Table 2. Pre-post objective scores for series of 5 TMS sessions (N=52)
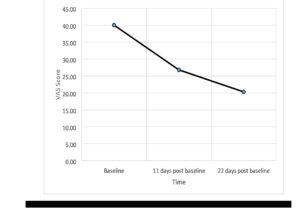
For the VAS there were 4 participants who did not complete the third time point leaving 48 participants (as they went into remission and were discharged before the 20th treatment). The VAS fell from 40.0 to 26.8 (11 days later) followed by 20.2 (a further 11 days later) (see Figure 1). Repeated measures ANOVA showed a significant reduction in VAS score [F(2,44)=11.3; p<.001, Eta2=.20] but no significant gender difference (p=.97), or impact of age (p=.86).
Outcomes by HAM6 Severity Ratings
Table 3 shows 69% of patients (36/52) achieved remission. Of the 19 patients in severe depression before treatment, 63.2% (12) achieved remission. Similarly, of those with moderate depression, 75% (21/28) achieved remission, as did 75% (3/4) of those with mild depression.
Table 3. Number of participants in each HAMD6 range before and after treatment
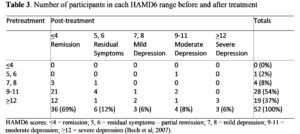
HAMD6 scores: <4 = remission; 5, 6 = residual symptoms – partial remission; 7, 8 = mild depression; 9-11 = moderate depression; >12 = severe depression (Bech et al, 2007).
Discussion
We chose the HAMD6 as it is preferred to the HAMD17 in terms of transferability, scalability and responsiveness (26,27,2); it is unidimensional (29,30,31) and is brief enough to be easily managed in a busy service clinic.
Our results (69% of MDD patients achieving remission) are (as expected) superior to those of controlled trials (4,5). Our patients were taking a range of antidepressants while those in controlled trials are medication free – evidence indicates different treatments may act synergistically (32). Our study provided what treating staff believed to be state of the art treatment; this would have been conveyed to patients, thus the placebo effect would be heightened. Slight treatment parameter differences between our study and the cited controlled trials may possibly (unlikely) have influenced the outcome. As mentioned, in Australia at the moment, the cost of TMS is not covered by the government or private insurers, so that in private practice, patients must be admitted to hospital to allow this cost to be recovered from the hospitalization rebate. Thus, when these people received TMS treatment for MDD, they were inpatients, which would also have contributed to their recovery. Finally, 35% had received TMS previously, and if TMS produces a beneficial effect at first treatment, it will do so at subsequent treatments. Thus, this 35% of patients were very likely to achieve substantial benefit.
Our results were also (as expected) superior to the 28% remission of the naturalistic outpatient study of Galletly et al (12). Due to commercial circumstances, we provided inpatient care – thus, for our patients, the stressors of everyday life were replaced by support and encouragement from trained clinical staff.
This was the first study (to our knowledge) to examine the effect of TMS on various levels of depression. For moderate and mild depression, remission was achieved in 75% of cases. In severe depression, remission was achieved in 63.2% of cases. This is important new information – that TMS is indicated in not only in mild and moderate, but also in severe MDD. Again, that 35% of our patients had previous experience of TMS is a complication.
Neither age nor gender impacted on outcome. The absence of an influence of age on outcome is consistent with Rostami et al (13). While the numbers in this study were small – 38 females and 20 males – the absence of a significant difference indicates that any actual difference must be minor. There were also no differences in symptom reduction in participants who had or had not had past ECT or TMS treatment, suggesting outcomes from acute TMS are not influenced by these prior treatments.
We used two objective metrics (HAMD6 and CGI) and the results were in agreement. We also used a subjective measure (VAS6). The results of the objective and subjective were also in agreement, which gives confidence in the findings. The graph of the VAS6 results [Figure 1] indicates an orderly decline in subjective depression. This is as anticipated, but has not been previously demonstrated (to our knowledge). We believe the VAS6 considered in this manner can illustrate/demonstrate the placebo response. Occasionally, a patient is encountered who manifests dramatic early improvement, which is lost by the 20th treatment – the VAS6 at three points in time can usefully quantify the good improvement midway through treatment which is lost by the end of treatment.
Figure 1. VAS Scores at baseline, 11 days post baseline and 22 days post baseline.
In current clinical practice, all patients who come to TMS have failed at least one or two trials of antidepressants, and satisfy the criteria for TRD. Thus, all of the patients in this study presented difficult clinical challenges, not simply those who, on this occasion, had a HDRS6 score in the severe range. The overall remission rate of 69% (albeit assisted by hospital admission, and that 35% of patients were known TSM responders) indicates TMS is a highly effective therapy by current standards.
TMS is effective in mild, moderate and severe depression. It should be available to inpatients and out patients in private and public settings.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interest: The authors have no conflicts of interest.
References
- Rush A, Kraemer H, Sackheim H, et al. (2006) Report by the ACNP Taskforce on response and remission in major depressive disorder. Neuropsychopharmacology 31(9): 1841-1853.
- Kessler R, Berglund P, Demler O, et al. (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA; 289(23): 3095–3105.
- Fava M (2003) Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 53(8): 649-659.
- O’Reardon J, Solvason H, Janicak P, et al. (2007) Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 62(11): 1208-1216.
- George M, Lisanby S, Avery D, et al. (2010) Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham controlled randomized trial. Arch Gen Psychiatry 67(5): 507-516.
- Berlim M, Van den Eynde F, Daskalakis Z (2013) Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized double-blind and sham controlled trials. Neuropsychopharmacology 38(4): 543-551.
- Leggett L, Soril L, Coward S (2015). Repetitive transcranial magnetic stimulation for treatment-resistant depression in adult and youth populations: a systematic literature review and meta-analysis. Prim Care Companion CNS Disord 17(6). doi: 10.4088/PCC.15r01807. eCollection 2015.
- Milev R, Giacobbe P, Kennedy S, et al (2016) Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the management of adults with major depressive disorder: Section 4. Neurostimulation treatments. Can J Psychiatry 61(9): 561-575.
- Perera T, George M, Grammer G et al (2016) The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul 9(3): 336-346.
- Levkovitz Y, Isserles M, Padberg F, et al. (2015) Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicentre randomized controlled trial. World Psychiatry 14(1): 64-73.
- Carpenter L, Janicak P, Aaronson S, et al. (2012) Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in a clinical practice. Depress Anxiety 29(7): 587-596.
- Galletly C, Clarke P, Carnell B, et al. (2015) A clinical repetitive transcranial magnetic stimulation service in Australia: 6 years on. Aust N Z J Psychiatry 49(11): 1040-1047.
- Rostami R, Kazemi R, Nitsche M, et al. (2017) Clinical and demographic predictors of response to rTMS treatment in unipolar and bipolar depressive disorders. Clin Neurophysiol 128(10): 1961-1970.
- Aguirre I, Carretero B, Ibarra O, et al. (2011) Age predicts log-frequency transcranial magnetic stimulation efficacy in major depression. J Affect Disord 130(3): 466-469.
- Conelea C, Philip N, Yip A, et al. (2017) Transcranial magnetic stimulation for treatment-resistant depression: naturalistic treatment outcomes of younger versus older patients. J Affect Disord 217: 42-47.
- Fidalgo T, Morales-Quezeda J, Muzy G, et al. (2014) Biological markers in noninvasive brain stimulation trials in major depressive disorder: a systematic review. J ECT 30(1): 47-61.
- Pridmore S, Bruno R, Turnier-Shea Y, et al. (2000) Comparison of unlimited numbers of rapid transcranial magnetic stimulation (rTMS) and ECT treatment sessions in major depressive episode. Int J Neuropsychopharmacol 3(2): 129-134.
- O’Sullivan R, Fava M, Agustin C, et al. (1997) Sensitivity of the six-item Hamilton Depression Rating Scale. Acta Psychiatr Scand 95(5): 379-384.
- Cowdry R, Gardner D, O’Leary K, et al. (1991) Mood variability: a study of four groups. Am J Psychiatry 148(11): 1505-1511.
- Guy W (1976) ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration.
- Paykel E (2008) Partial remission, residual symptoms, and relapse into depression. Dialogues Clin Neurosci 10(4): 431-437.
- Bech P, Lunde M, Bech-Andersen G, et al. (2007) Psychiatric outcome studies (POS): Does treatment help the patients? A Popperian approach to research in clinical psychiatry. Nord J Psychiatry 61 Suppl 46: 4-34.
- Kyle P, Lemming O, Timmerby N, et al. (2016) The validity of the different versions of the Hamilton Depression Scale in separation revision rates of placebo and antidepressants in clinical trials of major depression. J Clin Psychopharmacol 36(5): 453-456.
- Lee C-P, Liu C-Y, Hung C-I (2017) Psychometric evaluation of a 6-item Chinese version of the Hamilton Depression Rating Scale: Mokken scaling and item analysis. Asia Pac Psychiatry 9(3): e12287.
- Bachner Y, O’Rourke N, Goldfracht M, et al. (2013) Psychometric properties of responses by clinicians and older adults to a 6-item Hebrew version of the Hamilton Depression Rating Scale (HAMD6). BMC Psychiatry 13: 2.
- Timmerby N, Andersen J, Sondergaard S, et al. (2017) A systematic review of the clinimetric properties of the 6-item version of the HamiltonDepression Rating Scale (HAM-D6). Psychother Psychosom 86(3): 141-149.
- Hooper C, Bakish D. (2000) An examination of the sensitivity of the six-item Hamilton Rating Scale for Depression in a sample of patients suffering from major depressive disorder. J Psychiatry Neurosci 25(2): 178-184.
- De Carvalho Alves L, De Almeida Fleck M, Boni A, et al. (2017) The major depressive disorder Hierarchy: Rasch analysis of 6 items of the Hamilton Depression Scale covering the continuum of depressive syndrome. PLoS One 12(1): e0170000.
- Bech P (2015) The responsiveness of the different versions of the Hamilton Depression Scale. World Psychiatry 14(3): 309–310.
- Østergaard S, Bech P, Miskowiak K (2016) Fewer study participants needed to demonstrate superior antidepressant efficacy when using the Hamilton melancholia subscale (HAMD) as outcome measure. J Affect Disord 190: 842–845.
- Lee C-P, Liu C-Y, Hung C-I (2017) Psychometric evaluation of a 6-item Chinese version of the Hamilton Depression Rating Scale: Mokken scaling and item analysis. Asia Pac Psychiatry 9(3): e12287.
- Sackeim H (2016) Acute Continuation and maintenance treatment of major depressive episodes with transcranial magnetic stimulation. Brain Stimul 9(3): 313-319.